Consultation topics in pharmaceutical analysis and Quality Control

- Compendial requirements and procedures
- Analytical data integrity, evaluation and (continued) improvements of method performance
- Lifecycle management of analytical procedures,
identification and monitoring of relevant performance parameters,
assessment of changes - Method suitability and comparison
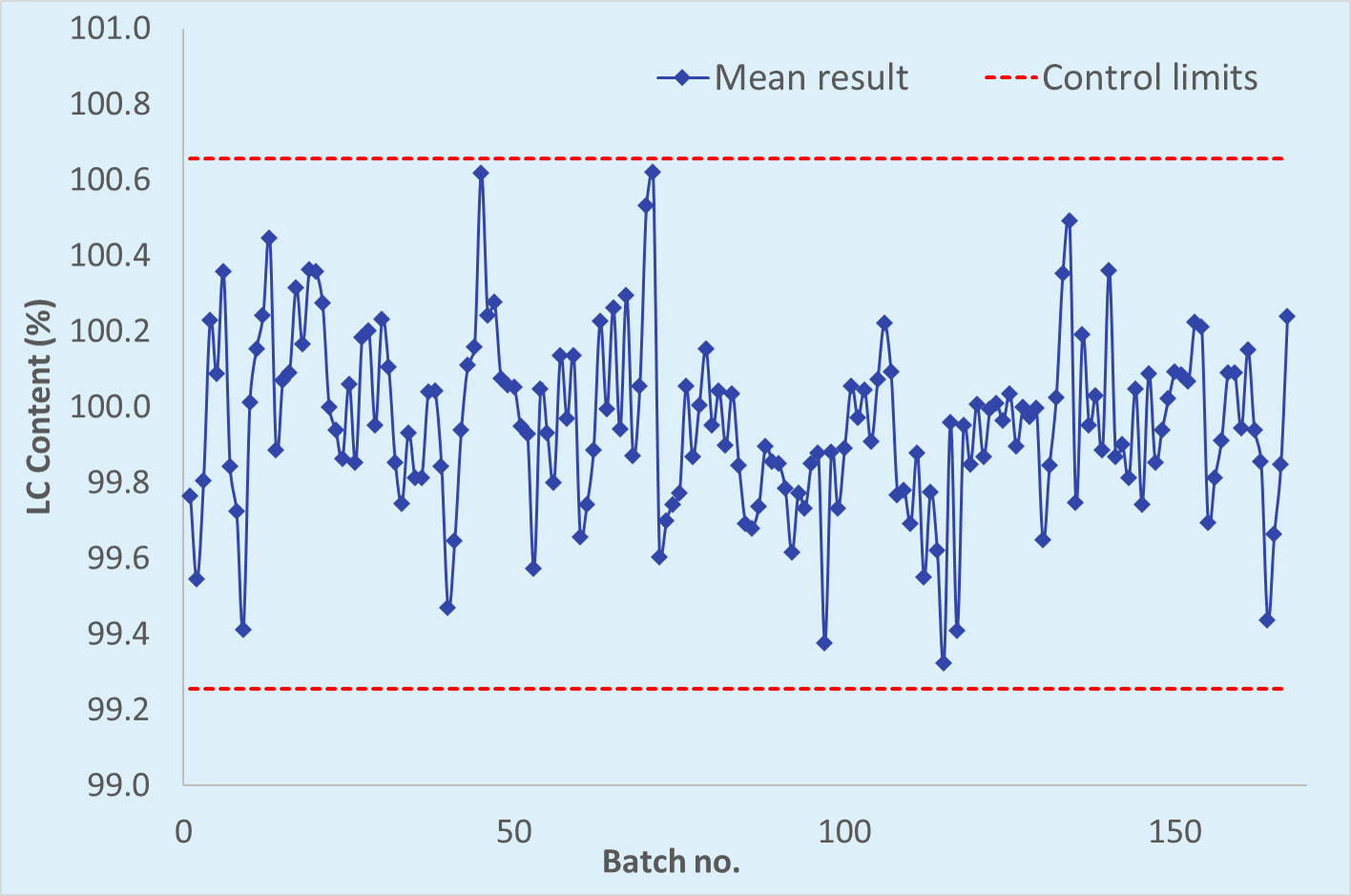
- Out-of-Specification (OOS) and Out-of-Trend (OOT) investigations,
establishment of alert limits - Practical statistics
- Reference standards
- Stability investigations including forced degradation studies (ANVISA)
- Validation in pharmaceutical analysis
- Verification of compendial procedures
- Transfer of analytical procedures
- Implementation or assessment/audit of quality systems with respect to the aforementioned topics
I would be delighted to support you, for example in planning or reviewing analytical studies, in deriving appropriate acceptance criteria, in statistical calculations, in assessing analytical results, or in generating or reviewing reports. In this way, you can benefit from my long-standing expert and management career in pharmaceutical analysis and Quality Control, as well as in various Working Groups.
I hope to have raised your interest and I am looking forward to get in touch with you. Please do not hesitate to contact me to discuss more details:
+49 151 / 28 76 11 66